Research
Research
The Beaudry group is broadly interested in natural products and creating methods for their synthesis. The natural products we target are from every major natural product class. Moreover, the reactions we develop are diverse in terms of mechanism; we have created new pericyclic, ionic, and radical reactions. Some themes of our research are described on this page. See also our publication list for more details.
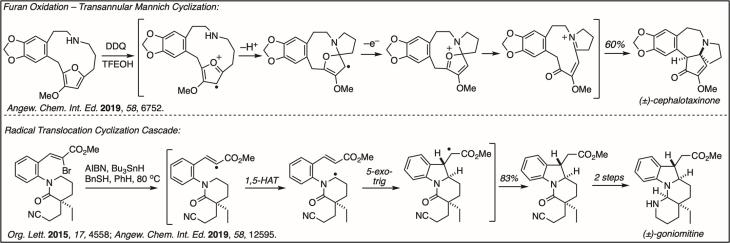
Cascade Reactions in Synthesis: We develop new complexity-building cascade reactions for synthesis. In the top example shown, an oxidative furan opening was followed by a Mannich cyclization to give the alkaloid cephalotaxinone. In the bottom example, we developed a radical translocation (or 1,5-HAT) followed by a 5-exo-trig cyclization for the synthesis of goniomitine.
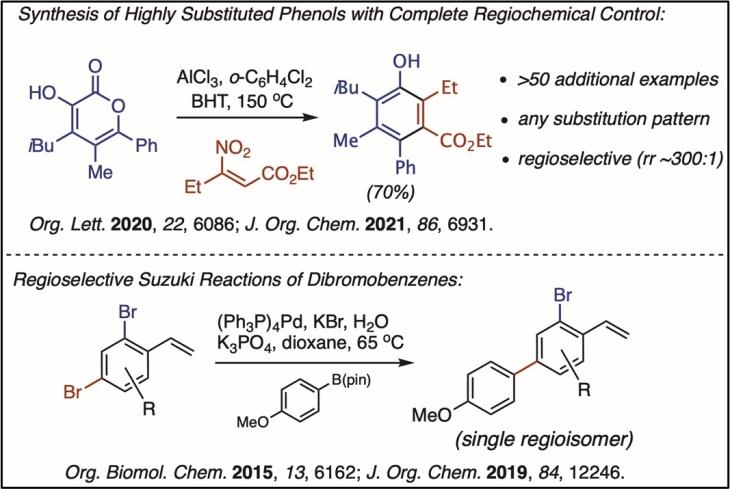
New Regioselective Reactions: Preparing aromatic molecules with predictable control of substituent regiochemistry is important for synthesis, particularly for pharmaceutical development. We discovered several new syntheses of aromatic molecules that give complete control over substituent regiochemistry. In the top example shown, hydroxypyrones react with nitroalkenes in a cycloaddition-elimination cascade to give phenols, even fully substituted phenols, with complete regiochemical control. The bottom example shows a regioselective Suzuki reaction of a dibromostyrene.
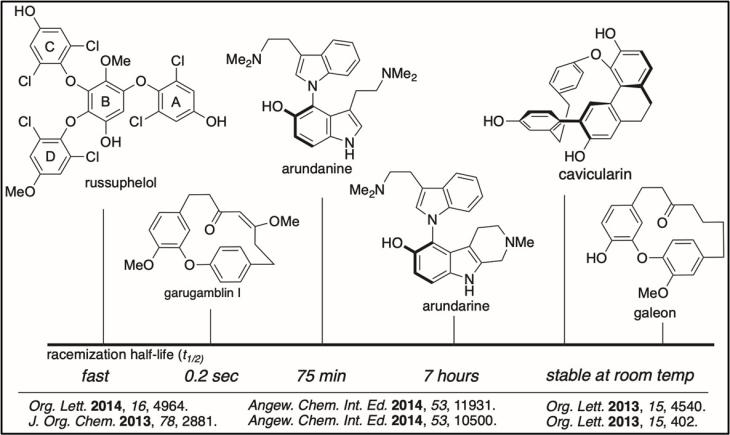
Investigating Natural Products with Conformational Chirality: We are interested in molecules that display molecular chirality without having sp3-hybridized stereocenters. It is our contention that many of these natural products have misunderstood chiral properties, because it is difficult identify chirality in the absence of stereogenic carbon atoms. For example, it was speculated that russuphelol was chiral; however, we found that it does not possess stable enantiomeric conformations, even at low temperature. Conversely, arundarine was believed to be achiral, but it can be isolated as a single enantiomer and racemizes slowly at room temperature (racemization half-life of approximately 7 hours).